Are you searching for possible treatment options for Metastatic prostate cancer?
Prostate Cancer (PCA) is the most common form of cancer diagnosed in men. While most patients respond well to surgery or radiation treatment, some develop advanced disease and become incurable. Prostate cancer is the second leading cause of cancer deaths in men.
The American Cancer Society estimates that over 30,000 new cases of prostate cancer were diagnosed in 2020 alone. Prostate cancer is usually treated by various methods including Surgery (prostatectomy), radiation therapy, hormonal therapy, chemotherapy, immunotherapy as well as targeted therapy which has been recently added to the list. In the early stages of cancer, these methods might give long-term remission but in advanced metastatic prostate cancer, they only help in shrinking the tumors instead of curing the disease.
There are two main types of treatment that are commonly used in advanced metastatic castration-resistant prostate cancer (mCRPC) i.e. chemotherapy and radiation therapy. Both methods not only kill the cancer cells but also affect the nearby healthy tissues and therefore, are not very effective against prostate cancer because they don't target the cancer cells only.
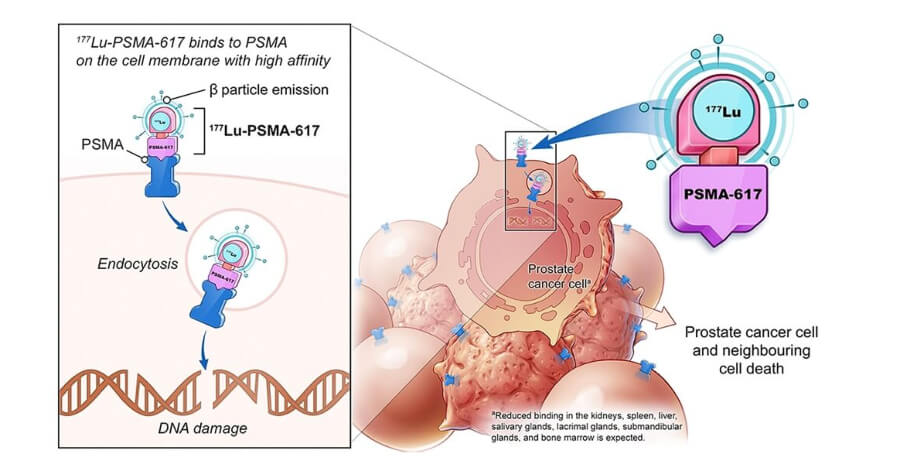
A new form of treatment called Lu-177 PSMA therapy has been developed to target prostate cancer cells, especially in the treatment of advanced metastatic castration-resistant prostate cancer. The drug is injected into the bloodstream, attaching itself to the cell surface protein known as PSMA. This allows the Lu-177 PSMA to damage the cell by delivering its payload of radiation directly to the cancerous cells.
What is Lutetium-177 PSMA Therapy?
Lu-177 PSMA therapy uses a radioactive isotope of lutetium (Lu), found naturally in soil. In this case, the radioisotope used is lutetium-177 (Lu-177). It emits high-energy gamma rays that can damage DNA inside the cancer cells.
This type of therapy is referred to as radiopharmaceutical therapy (RPT) and involves using a radioactive substance that targets specific molecules on the surface of cancer receptors. Once the radiolabeled compound reaches the tumor site, it binds to receptors on the surface of cancerous cells. Once attached, the compound releases a small amount of radiation eventually causing the death of cancer cells from the effects of radiation exposure
Who Qualifies for lutetium-177 PSMA Treatment?
The following criteria are required to qualify for Lu-177 PSMA:
- You are 18 years old or older.
- You do not have another serious illness or condition I.e. kidney failure, liver failure, etc.
- You are suffering from advanced metastatic castration-resistant prostate cancer
- You are not responding to other treatments (i.e. hormonal therapy and chemotherapy)
If you have received treatment with Xofigo or other types of radiation therapy, for prostate cancer, you should wait 2 weeks after completion of these therapies before participating in lutetium-177 PSMA therapy.
Your doctor decides that the benefits outweigh the risks associated with the procedure.
How Does Lu-177 PSMA Therapy Work?
The lutetium-177 PSMA is administered intravenously through a catheter. Because the Lu-177 PSMAs attach themselves to specific proteins present on the surfaces of prostate cancer cells, the therapy works only when the patient's tumors express Enough amount of PSMA receptors—which often occurs in late-stage prostate cancer.
Once the Lu-177 PSMA molecule enters the body, it travels throughout the bloodstream until it finds an area with active prostate cancer growth where it gets attached to the PSMA receptor. Once attached, it delivers its payload of radiation directly into the nucleus of those cells. This means the radiation will be absorbed primarily by cancer cells and not by surrounding healthy tissues.
How is the program carried out?
During the visit, you will be asked for a complete medical history, including all medications taken, current medication, allergies (if any), and family medical history. A physical examination is performed, followed by a Gallium 68 (Ga-68) PSMA PET CT scan that helps in determining the amount of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer. If your doctor suspects a problem, they will order blood tests to check levels of calcium, creatinine, liver function, etc. Your urine will be tested for sugar content and the presence of albumin. An ECG (Electrocardiogram) is done to determine heart rhythm. Blood pressure is checked. You will need to sign consent forms before receiving any procedures.
Before Procedure
Most people have no special preparation before undergoing this type of treatment. But it is recommended that patients drink plenty of fluids for several days before receiving the treatment. Additional precautions include avoiding alcohol consumption and not taking aspirin and anticoagulants.
During Procedure
The patient will lie down on an examination table during the treatment procedure. Then medical personnel will inject the carrier containing the isotopes through a needle into the patient's arm. Afterward, the patient will move onto another exam table where they will remain for about 1 hour. During this time, the isotopes travel throughout the patient's body.
Lutetium-177 PSMA infusion typically lasts between 30 minutes and 90 minutes.
After Procedure Recovery Time & follow-ups
After having Lu-177 PSMA Radiotherapy, most patients return home within 48-72 hours. They should avoid strenuous activities for 24 hours following the treatment.
The following therapy can take place in 6-8 weeks. A patient may be recommended to have three treatments, but a single treatment may be enough.
How is the effectiveness of the therapy measured Post Treatment?
The effectiveness of therapy is measured by performing the below investigations:
- Full body scintigraphy– before discharge from the hospital
- PSA (in 4-8 weeks)
- Imaging studies – PSMA PET CT scan, CT scan, Bone Scan – 3-12 months post-treatment
Evaluation of response to treatment:
After completing the scheduled number of administrations of the labeled peptide, measurements are taken to determine whether there was any change in the PSA level. In addition to standard quality assessments, an evaluation of the size of lesions by Ga68 DOTANOC positron emission tomography/computed tomography scanning is performed at each three months interval after the last administration of the radioisotope.
Prognosis
Approximately 80% of men who undergo Lu-177 PSMA radioactive therapy respond positively to the treatment. However, some patients may require an additional cycle of treatments.
Side Effects:
Although Lu-177 PSMA therapy is considered safe as the dose of Lutetium-177 radiation is well calculated for each patient before the therapy but still, some side effects can be expected including:
- Increased risk of bleeding
- Nausea
- Dry Mouth
- Fatigue
Other possible side effects include fatigue, fever, headache, bone marrow suppression, skin rash, and low blood counts. Patients should inform their physicians if they develop any symptoms that could worsen these conditions.
Advantages of Lu-177 PSMA Over Other Treatments
Compared to other forms of treatment like chemotherapy and external beam radiation, Lu-177 PSMA has several advantages:
- It doesn't cross the blood-brain barrier and therefore won’t affect the central nervous system.
- It is effective even when all other treatments failed
- It can be used in advance metastatic castration-resistant prostate cancer
- There are no severe side effects associated with this form of treatment.
- PSA levels tend to drop rapidly after starting treatment
Disadvantages of Lu-177PSMA Therapy
While there are many benefits to using Lu-177 PSMA over existing therapies, there are also potential drawbacks. These include:
- It may take multiple administrations of the radiolabeled agent to achieve maximum results.
- Risks associated with the administration of the radioactive material must be evaluated before each treatment.
- Like all forms of radiation therapy, patients may experience some discomfort during treatment.
Cost of Lu177 PSMA therapy in Germany
The cost for treatment of the last stage of prostate cancer with Lutetium-177 PSMA therapy varies depending upon which hospital and the treating doctor.
PSMA therapy with Lutetium 177 in specialized hospitals ranges between 8000- 16,000 euros. This amount covers the cost of the initial clinical and laboratory examination, the PSMA therapy itself, the follow-up examinations, the hospital stay, and the elaboration of recommendations for future treatment.


Comments