Are you searching for the latest treatment options for Metastatic castration-resistant prostate cancer?
Prostate cancer is the second most common cancer diagnosed in American males today. More men die from complications associated with prostate cancer and metastatic castration-resistant prostate cancer (mCRPC) than with lung cancer. Prostate cancer disproportionately affects African Americans and American Indians/Alaska Natives and black men are 1.3 times more likely to develop prostate cancer than white men. In 2005, prostate cancer was the third leading cause of death among U.S. men where African Americans had the highest incidence rate of prostate cancer compared to all other ethnic groups.
The FDA-approved recently approved Actinium-225 PSMA therapy that uses radiolabeled monoclonal antibodies to target PSMA-positive tumors. In contrast to conventional external beam radiotherapy where the whole organ or at least a big part of the organ is affected, the Ac-225 PSMA therapy allows doctors to deliver radiation directly to the tumor without affecting the nearby tissues. This therapy is recommended for patients who cannot tolerate other treatments or whose disease progresses even after receiving all other kinds of therapies. It can also treat patients with bone metastases, lymph node involvement, or visceral metastases.
According to a research study conducted by the University of Texas MD Anderson Cancer Center. Patients who had failed previous hormone therapy were randomly assigned to receive either Ac-225 PSMA or a placebo. After six months, the researchers found that patients receiving Actinium-225 PSMA showed significantly improved overall survival compared with those who received a placebo.
What is Ac-225 PSMA therapy?
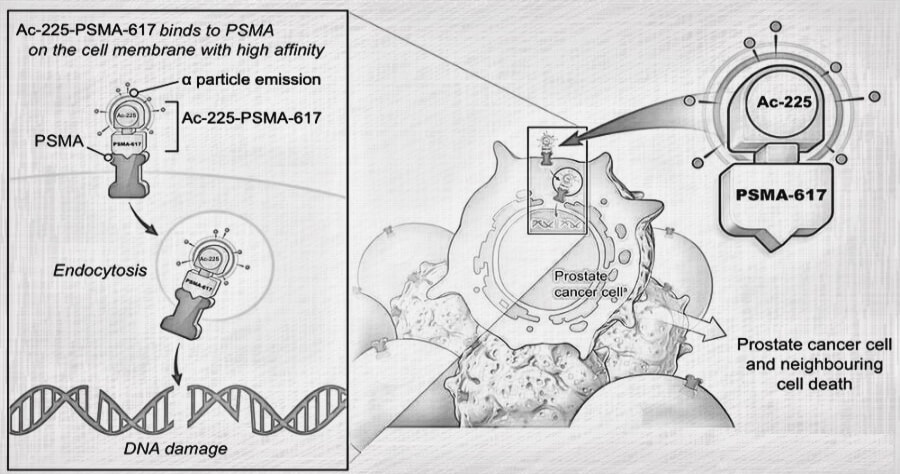
Ac-225 is a targeted α-particle therapy in which a radiolabeled anti-prostate specific membrane antigen (anti-PSMA) monoclonal antibody Actinium-225 (Ac-225) binds selectively to human PSMA-expressing cells and delivers radiation to these tumor cells damaging their DNA and eventually causing cells to die via apoptosis
What should I know before undergoing Actinium-225 PSMA therapy?
Before undergoing the treatment with Actinium-225 PSMA therapy, the treating doctor will assess the extent of the spread of the prostate cancer for which he will need to see recent images from a Ga68-PSMA PET CT scan.
If you decide to pursue Actinium-225 PSMA therapy, here are some things to keep in mind:
- Ac-225 is based on two independent factors, the PSMA level (Assessed by Ga68-PSMA PET CT scan) and your Gleason score. A higher PSMA value indicates a greater likelihood that your cancer will respond to the therapy, while a higher Gleason score suggests a worse prognosis.
- Treatment of the last stage of prostate cancer with Actinium-225 PSMA therapy is usually recommended if the patient is resistant to other therapies, especially Lu-177 PSMA therapy.
How does this therapy work?
Once the Ac-225 PSMA complex attaches itself to the PSMA receptor on the prostate cancer cell, it emits strong α-particles that damage the double-stranded DNA of the cell causing cell death.
Unlike conventional chemotherapy and radiation therapy, which damage healthy cells as well as cancerous ones, radioactive antibody therapies like Actinium-225 PSMA selectively kill only malignant cells expressing PSMA receptors.
Before Procedure
Your health care provider will take a detailed medical history and routine baseline investigations and explain what to expect during your procedure. You'll likely be asked to sign a consent form that allows your physician to perform tests and give you medicine.
During the procedure
The patient will lie down on an examination table during the treatment procedure. Then the doctor will infuse the carrier containing the isotopes through a needle into the patient's arm which takes approximately 30-90 minutes. Afterward, the patient will move onto another exam table where they will remain for about 1 hour. During this time, the isotopes travel throughout the patient's body.
After the Procedure
It’s important to drink plenty of fluids following treatment. It would be best to take it easy for several days following the procedure. Do not lift anything heavier than 10 pounds. Avoid sexual activity until advised otherwise by your doctor. Report any unusual bleeding or bruising immediately.
The patient is usually kept under observation for 24-48 hours after which full-body scintigraphy is performed to estimate the absorbed doses for normal organs and tumor lesions and if the values are under normal range, the patient is discharged.
Follow-ups
Follow-ups are conducted every 4-8 weeks initially with PSA and if a rise is detected, a PSMA PET CT scan is performed.
The number of treatment cycles depends on the extent of your disease and response to therapy. Patients who have early-stage cancers typically receive lesser cycles; late-stage or end-stage cancers typically receive more cycles over a period of 2 years. Each dose causes radiation damage to cancer cells.
How is the effectiveness of Ac-225 PSMA therapy measured Post Treatment?
The effectiveness of therapy is measured by performing the following investigations:
- Full body scintigraphy– before discharge from the hospital
- PSA (in 4-8 weeks)
- Imaging studies – 68 Ga PSMA PET CT scan, CT scan, MRI, Bone Scan – 3-6 months post-treatment
Prognosis
The prognosis depends upon the spread of cancer and the initial response to the treatment.
According to a clinical study, In patients who responded to Ac-225 PSMA therapy, the median time to progression and overall survival was 12.5 months and 32.6 months respectively and the Median PSA doubling time was 11 months. While in non-responders, the median time to progression was 1.4 months, and overall survival was 7.3 months.
The response to radiotherapy is dependent on the initial volume of disease burden, the patients with lower disease burden had more durable responses.
What are the typical Side Effects of Actinium-225 PSMA Therapy?
The most common side effect is anemia (low red blood cell count) and Dry mouth. Other side effects include a decrease in iron, calcium, magnesium, phosphate, or vitamin B12.
Possible complications associated with radiation exposure during this type of radiation therapy include:
- Cardiovascular Disease (heart disease)
- Kidney Problems
- Nerve Damage
- Radiation Myelopathy (a condition that causes nerve damage leading to paralysis)
- Sexual Dysfunction
But all of the above-mentioned side effects are very rare and are seen in < 1% of cases.
What about the safety of Ac-225 PSMA therapy?
Because Ac-225 PSMA therapy was safe in clinical trials, the Food and Drug Administration approved its use in men with advanced prostate cancer. However, this treatment should not be given to anyone if they have an active infection because of the risk of serious infections. Also, people taking certain medications, including blood thinners and antihistamines, should not take this therapy.
Cost of Actinium-225 PSMA therapy in Germany
The cost for treatment of mCRPC with Ac-225 PSMA therapy varies depending upon which hospital and the treating doctor.
PSMA therapy with Actinium 225 in specialized hospitals ranges between 8,000- 15,000 €. This amount covers the cost of the initial clinical and laboratory examination, the PSMA therapy itself, the follow-up examinations, the hospital stay, and the elaboration of recommendations for future treatment.


Comments